VPD is critical to the vitality of your plants. If you’re going to make the most of the grow room you’ve invested so heavily in, you need to fully understand the concept of VPD.
You can learn about these concepts in the time it takes to read a few paragraphs! If you’ve read an article that mystifies the concept of VPD, or tries to convince you that it takes a special engineering degree to fully understand VPD, then you’ve been misled. VPD is an involved topic, but that doesn’t mean you can’t understand the concept and its implications for your grow room.
We learned in grade school that plants need water. On a day-to-day basis, we see sprinklers watering plants in gardens and yards. But did you know that 99% of the water that enters the root system ends up leaving the plants through holes in the leaves? In fact, it’s more accurate to say that the air sucks water out of plants than to say that the plants suck water out of the ground! And this whole effect happens because of the principles behind VPD.
In the context of controlled environment horticulture, the definition of VPD is, quite simply:
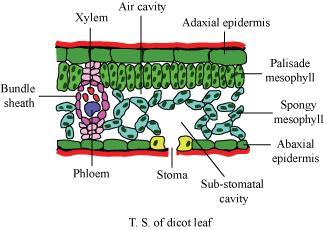
Vapor Pressure Deficit (VPD) is the difference between the vapor pressure measurement in the substomatal pocket (that microscopically small pocket of air inside the leaf behind the stomata) and the vapor pressure measurement of air surrounding the leaf. Image courtesy of https://www.toppr.com/ask/question/describe-the-internal-structure-of-a-dorsiventral-leaf-with-the/
Understanding Vapor Pressure
There’s quite a bit of talk in the industry about vapor pressure deficit, but it’s not often folks dive specifically into vapor pressure. Let’s get to the bottom of that now:
Vapor Pressure is defined as the force acting on a liquid surface that keeps the liquid in a liquid state instead of letting it evaporate off as a gas/vapor.
We all know that when water boils, it is quickly converting from its liquid state to a gaseous state. Boiling is a special case where the water converts to a vapor below the surface of the water (those are bubbles of steam that start at the bottom of the pot and work their way to the top). At any temperature less than the boiling point of that liquid, the only water that converts to vapor will be molecules at the surface of the liquid. From here on out, we’re going to use liquid and vapor in reference only to water.
Here’s an easily understandable example. If you take a glass of water and leave it alone, untouched, for 24 hours on your desk or in a windowsill, the level of the water will have gone down when you return. You didn’t boil the water away, right? Instead, the water turned into a vapor (the water e-vapor-ated) because there was not enough vapor pressure keeping those water molecules in place.
If the air in the room was, say, 75F and had a low relative humidity (30%), more water would have evaporated in that 24 hours than if the room was 75F and had a high relative humidity (70%). At a given temperature of air, the vapor pressure is higher when the relative humidity is higher. Remembering our definition then, a high vapor pressure will act to keep the water in a liquid state, instead of letting it evaporate.
What causes this pressure? It turns out that the pressure on liquid water to stay a liquid is actually caused by… (drumroll please)
Water vapor. In the air, in between all those molecules of oxygen, nitrogen, argon and carbon dioxide that make up most of our atmosphere, are molecules of water. Sometimes air has more water in it, sometimes air has less water in it, but there is a finite amount of water in the vapor state mixed in with the other molecules. The measure of water in vapor form that we find most useful here is “Relative Humidity” – at a specific temperature, relative humidity describes the relative amount of water-in-air compared to the greatest amount of water-in-air that could exist in the vapor form (it is expressed as a percentage).
- If there is only a small amount of water in the air compared to the maximum amount of water vapor that could exist (low relative humidity), there is very little force pushing back on any surface of liquid water to keep those water molecules in liquid form. Water evaporates quickly if the relative humidity is low.
- If there is a lot of water in the air compared to the maximum amount of water vapor that could exist (high relative humidity), there is a lot of force pushing back on any surface of liquid water. Water evaporates slowly when the relative humidity is high.
- And if the air is saturated with water (100% relative humidity) – there literally is NO more water that the air can hold. If you took your glass of water from the previous example and placed in a room at 100% relative humidity, the water level would not have gone down at all after 24 hours!
******** Side Note: In the temperatures we care about it, water makes up only a very small fraction of the total molecules of air, regardless of its relative humidity percentage. For example, at 80F and 50% Relative Humidity, water vapor makes up 1.1% of air. At 80F and 100% Relative Humidity, water vapor makes up 2.2% of air. The other 98% is nitrogen, oxygen, argon, CO2. ********
Bringing it Back to Plants
Inside a leaf, the walls of the substomatal cavity are covered in a thin layer of liquid water. This cavity can be thought of as a very small, wet cave, with just a tiny opening from which anything can escape. It turns out that the moisture content of the substomatal cavity is at 100% relative humidity. The air inside the substomatal cavity is at the highest possible vapor pressure for that temperature of air.
Earlier, I told you that at 100% relative humidity, moisture won’t evaporate from the surface of liquid water. So how is it that the water goes from the liquid form to the vapor form in the process of plant transpiration?
The primary cause of transpiration and effective water movement through plants is to ensure that the vapor pressure outside the leaf is lower than the vapor pressure inside the leaf. If we get the surrounding environment right, we can ensure that just the right amount of water evaporates inside the leaf and gets sucked out of the stomata. Gases tend to move from high pressure to low pressure, and water vapor is no exception.
Let me restate that for emphasis: A low vapor pressure outside the leaf (lower relative humidity outside the leaf than inside the leaf) will literally PULL the water out of the stomates on a plant. In a very real way, plants don’t expel water vapor from the stomata, it’s the atmosphere that sucks the water vapor out. And every molecule of water that gets sucked out of the plant and into the environment is one more molecule of water that needs to be brought back to the leaf through effective irrigation.
It may not be readily apparent, but the speed at which water vapor is sucked through stomata has a direct impact on plant vitality. Those stomata are the gateways for all the gas transfer required for plant metabolism. If you’re blasting water vapor out of the stomata at too high a rate (with very low relative humidity in your cultivation room), then you are unable to diffuse carbon dioxide from the environment into the stomata in the opposite direction, and CO2 is an extremely important molecule for the process of photosynthesis. On the other end of the spectrum, if you have a very high relative humidity in your cultivation room, then you are slowing the evaporation of water inside the plant (bonus – this also means you need to water less) and CO2 can very easily diffuse into the substomatal cavity (double bonus – the plant has more access to plant food!) That sounds like a very good thing, but typically this occurs at a humidity level inside the room where pathogen proliferation is a real threat to plant vitality. What a delicate balance between risk and reward!
Now you know the “what” of VPD, stay tuned for the next article in this series that will discuss the “how” of measuring and using VPD as an effective tool in your cultivation space.